- Category:
- News
How does hydrogen work in a fuel cell electric vehicle?
Hydrogen’s ability to store and release energy make it an ideal vehicle fuel. It can be used to fuel vehicles in two main ways:
- Burned with oxygen in an internal combustion engine (ICE) like conventional fossil fuels. This is being considered mainly for larger forms of transport like aviation and marine.
- Combined with oxygen in an electrochemical device called a fuel cell to provide electrical power to a vehicle.
Fuel cell vehicles
Fortunately, hydrogen is very well suited to a fuel cell that converts its energy to electrical energy.
Fuel cells are more efficient than an ICE because they do not burn the fuel.
Instead, a sequence of electrochemical reactions catalysed by special materials (catalysts) separates the O2 and the H2 and combines them into water (H2O), generating electricity and heat, in a process more efficient than combustion.
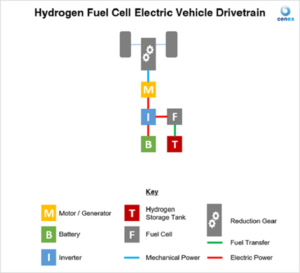
A vehicle that uses hydrogen in a fuel cell to generate power, known as a Fuel Cell Electric Vehicle (FCEV), comprises a fuel cell stack (multiple fuel cells combined to generate sufficient power to propel the vehicle), hydrogen tanks, battery, power electronics, and electric motor.
Insight: An Introduction to Hydrogen Vehicles and Refuelling Infrastructure
The workings of a fuel cell electric vehicle are summarised below:
- Hydrogen gas from the tank is supplied to the fuel cell stack.
- Air is also supplied to the fuel cell stack.
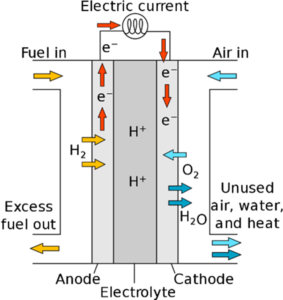
- In the fuel cell, hydrogen is converted to a proton and an electron. The electron is drawn out of the cell as electrical current, whilst the proton can travel through the membrane toward the cathode. The oxygen (from the air) reacts at the cathode with the protons and electrons to form heat and water.
- Electricity generated through the reaction is supplied to the electric motor and/or battery. The battery acts as an energy store, providing additional energy to the vehicle for acceleration events, and can also capture regenerative energy from braking to improve vehicle efficiency.
- The motor provides power to the wheels and the waste material is emitted from the vehicle via the exhaust – in this case, water!
Efficiency of a FCEV
A fuel cell’s efficiency (the amount of energy extracted from the fuel) is about 90%, however, in a vehicle it is tough to make use of all of the fuel cell’s efficiency.
Approximately half of the energy the fuel cell releases is low-temperature heat which in a passenger car, is challenging to make use of this low-grade heat reliably.
Therefore, in a passenger car, a fuel cell’s working efficiency is closer to 45% when powering an electric motor.
Take a free online course: An Introduction to Low Emission Road Transport
By the time we start thinking about overall efficiency of transferring the energy to the wheels, a FCEV passenger car’s total efficiency is closer to 30%.
As a ‘rule of thumb,’ you can assume that a FCEV will always be around twice as efficient as an equivalent ICE vehicle.
As well as better efficiency, the electrochemical operation of a fuel cell means that that FCEVs are much quieter than ICE vehicles, similar to EVs, and, crucially, their only emission is water, which offers a compelling advantage over ICE vehicles in achieving air quality and carbon reduction targets.
Cenex and hydrogen
Cenex has over 15 years’ experience in fuel cell technology and refuelling infrastructure, and is involved in hydrogen research projects, large scale demonstrations, and private consultancy.
The ZEFER project is deploying 180 FCEVs across three European capital cities (London, Paris and Brussels) in taxi, private hire and emergency service applications, to demonstrate the technical readiness and suitability of hydrogen transport.
There are also over 25 vehicles on the road as a result of the HyTrEc project to kickstart the hydrogen economy in the North Seas Region, and Cenex monitor how effectively those vehicles and refuelling stations work together.
Get in touch to see how you can use hydrogen transport to lower your emissions.